Conformation of polymers
Conformation is the position that each atom of the polymer takes on at a certain given time. In particular the position of atoms on adjacent carbons along the polymer chain, also called backbone, influence what positions that are preferred. The more electronegative and bulky atoms or side groups the more they avoid their proximity. In Figure 4 this is illustrated for a low molecular organic compound.
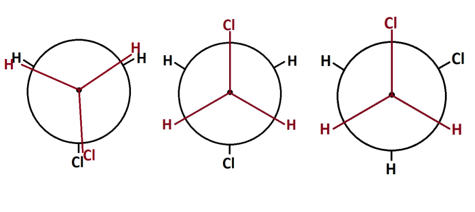
Figure 4. Newman projection of 1,2 dichloroethane molecule in different conformations
© Anders Persson, University of Borås
The likelihood of taking on the staggered position to the left in Figure 4 drops since a significant energy barrier must be overcome in order to rotate a whole revolution around the two covalently bonded carbons of the backbone. Transferred to a polymer this would give long ranging effects on the preferred overall shape of the whole polymer chain. If a polymer is free to find its preferred conformation it will try to reach its most disordered state. This is governed by the Second law of thermodynamics, which states that nature always moves towards higher entropy. Entropy in polymers can be interpreted as degree of disorder and is one of the major factors that control the behaviour of polymers.
The polymers are constantly moving around. This is called Brownian motion. The higher the temperature the more vigorously the movement becomes. It is just like gas pressure that raises with temperature since the gas molecules increase their velocity with temperature.

All efforts have been made to ensure materials created by the EDU comply with current accessibility guidelines (JISC: Support for learners with disabilities).
If further assistance is required with accessibility matters please contact the student support section in your academic partner UHI: Accessing learner support.
We welcome any comments on how to improve this unit. Please feel free to pass these on at any time.
If you have any difficulty viewing this resource please contact EDU (edu@uhi.ac.uk) with:
- the name of the resource;
- a description of the problem (please give as much detail as possible);
- the section of the resource where the problem occurred;
- your internet browser (you can check your browser version at: http://detectmybrowser.com/).
UHI provides links to external sources of information and may refer to specific Web sites, products, processes or services within this resource. Such references are examples and are not endorsements and whilst every effort is taken to ensure the accuracy of information provided UHI is not responsible for any of the content or guidance. You are advised to exercise caution.
Audio
Video
Reading
Download
Information
External link
Activity
Question
Asterisk
Discussion
Collaboration
Reflection/journal/log
History
Pause for thought
Download a copy of this resource in PDF format.
You can also print individual pages by printing directly from the browser.