Addition polymerization
During addition polymerization monomers are added to the growing polymer one by one. It has three distinctive steps; initiation, propagation and termination. An initiator is formed that attacks a monomer. Depending on the chemical nature of the monomer the initiators may be a radical, a cation or anion. The electronegativity of the monomer determines what initiator is stabilized. If the monomer has substituents or side groups they govern the preferred polymerization mechanism.
Radical polymerization was the first commercially utilized of the three and it is still used to make the big bulk plastics such as Low-Density Poly(Ethylene), LDPE, PVC, Poly(Styrene), PS and Poly(Methyl methacrylate), PMMA. Heat, radiation or chemical reactions generate two radicals by scission of a labile covalent bond. The radicals then attack double bonds of the two monomers that form monomer radicals that readily attack new monomers. This propagation phase is usually very rapid and short and ends by termination where the radical is extinct by some kind of termination reaction.
Two radicals may react with one another, i.e. combination termination, resulting in one long polymer. The two free electrons may also end up at one of the two radicals to give two polymers where one of them has a double bond at its end. This is called disproportionation. Different polymers are more or less prone to either of the two termination processes. Another termination process of a growing radical is if it is transferred to another species; an initiator, a monomer, another polymer or a solvent molecule. These are all called chain transfer reactions. Thus the polymers' molar mass, molar mass distribution and linearity are determined by their relative rate constants and how they compete with combination and disproportionation. In case of chain transfer to a polymer, i.e. reaction along its backbone it generates a branching point, something that is common for e.g. LDPE. For LDPE the active site may also jump along the growing polymer, so called back-biting, causing further branching.
Task
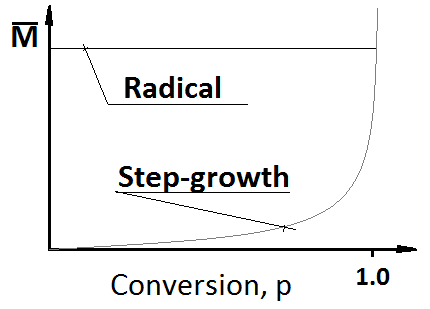
Draw a graph that shows how the molar mass increases with conversion for step wise and radical polymerization.
Draw a graph that shows how the molar mass increases with conversion for step wise and radical polymerization.
Answer: The graph shows how the mean molar mass increases for the polymeric molecules that are formed as a function of conversion for radical and step-grown mechanisms.
© Anders Persson, University of Borås

All efforts have been made to ensure materials created by the EDU comply with current accessibility guidelines (JISC: Support for learners with disabilities).
If further assistance is required with accessibility matters please contact the student support section in your academic partner UHI: Accessing learner support.
We welcome any comments on how to improve this unit. Please feel free to pass these on at any time.
If you have any difficulty viewing this resource please contact EDU (edu@uhi.ac.uk) with:
- the name of the resource;
- a description of the problem (please give as much detail as possible);
- the section of the resource where the problem occurred;
- your internet browser (you can check your browser version at: http://detectmybrowser.com/).
UHI provides links to external sources of information and may refer to specific Web sites, products, processes or services within this resource. Such references are examples and are not endorsements and whilst every effort is taken to ensure the accuracy of information provided UHI is not responsible for any of the content or guidance. You are advised to exercise caution.
Audio
Video
Reading
Download
Information
External link
Activity
Question
Asterisk
Discussion
Collaboration
Reflection/journal/log
History
Pause for thought
Download a copy of this resource in PDF format.
You can also print individual pages by printing directly from the browser.