Amorphous polymers and the glass transition
The glass transition is when a material changes its conformational mobility drastically. For polymeric materials it takes place at a certain temperature. This temperature, the glass transition temperature, Tg, is linked directly to a coordinated conformational mobility of the polymer back-bone. It has been suggested that it is 20 to 50 repeating units that are thermally activated. This transition can take place in materials without ordered, so called amorphous materials. If the amorphous material consists of a single phase it will be transparent. In semi-crystalline polymers that consist of both crystalline and amorphous phase, the amorphous phase will still have a glass transition. An amorphous thermoplastic will change its Young’s modulus about three decades during its glass transition.
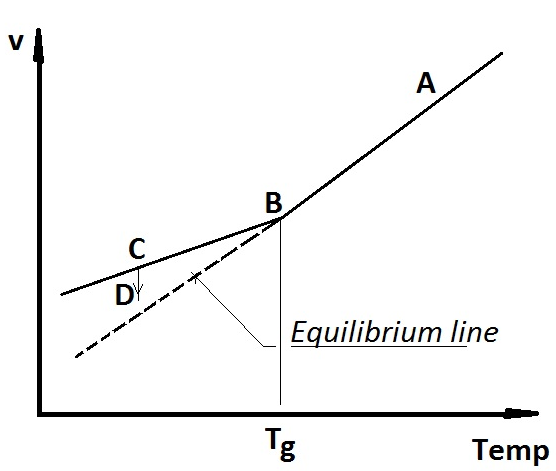
Figure 7. Schematic graph of specific volume - temperature for an amorphous polymer.
© Anders Persson, University of Borås
Each polymer has its own unique Tg. The glass transition is not a primary phase transition, such as melting or vaporisation, hence there is no phase transition heat involved. This makes Tg determination difficult. Changes of physical properties such as shrinkage/expansion, permeability, mechanical properties or cohesive energy density have to be studied. Figure 7 shows how a schematic amorphous material behaves upon cooling from A to B where the shrinkage alters pattern at the Tg. The shrinkage continues at a lower rate from B to C where the cooling is halted. If the temperature is kept constant for prolonged time the material will densify (C to D) since it will try to become as disordered as possible. The atoms will also come closer together. Both these driving forces move the material towards the equilibrium line, which is a continuation of the shrinking line above Tg. The space for conformational mobility decreases. This is often referred to as free volume reduction, which is called physical ageing.
If the atoms that make up the polymeric material come closer to one another it will also give more intermolecular bonds per volume unit, also called the cohesive energy density. Furthermore, the temperature is altered during the measurements and then changes in cooling/heating rate are coupled to the measurement methodology and maybe also other aspects of the method. For instance, the kink in the shrinkage curve of Figure 7 will move to a lower temperature in case it gets more time to take on a more disordered state and higher cohesive energy density. To make it even more complicated upon heating the sample that was slowly cooled will register with a higher Tg upon heating since its conformational mobility is restricted by its lower free volume. Hence, text book data on Tg‘s can scatter quite significantly.
Polymer constitution is the determining factor for the actual glass transition temperature of a particular polymeric material.
| Table 1. Constitutional factors of the polymer repeating unit that: | |
|---|---|
| raise the Tg | reduce the Tg |
|
|
Besides the factors in table 1 there are also a number of parameters that influence the conformational mobility of the polymeric material. The extreme of intermolecular attraction is when they have turned intramolecular, i.e. formation of covalent cross-links in-between polymer molecules. A similar extreme in terms of conformational mobility reduction due to intermolecular attraction is when a polymer crystalizes. Crystallization may take place both of the polymer back-bone or if the side groups are long enough to form crystalline domains by themselves. High molar mass also raises the Tg due to the relative absence of polymer end-groups that facilitate conformational mobility. Low molecular compounds absorbed by the polymeric material will reduce the Tg. They will work as internal grease and are often referred to as plasticizers. In PVC this is utilized to turn a rigid material into something rubberlike.
Task: Sort the following polymers in terms of increasing glass transition temperature.
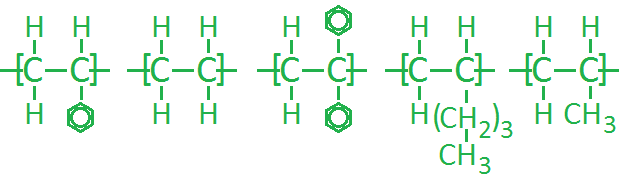
Suggested order with numbering from left to right:
2-4-5-3-1
2-5-4-3-1
2-5-4-1-3
2-4-5-1-3

All efforts have been made to ensure materials created by the EDU comply with current accessibility guidelines (JISC: Support for learners with disabilities).
If further assistance is required with accessibility matters please contact the student support section in your academic partner UHI: Accessing learner support.
We welcome any comments on how to improve this unit. Please feel free to pass these on at any time.
If you have any difficulty viewing this resource please contact EDU (edu@uhi.ac.uk) with:
- the name of the resource;
- a description of the problem (please give as much detail as possible);
- the section of the resource where the problem occurred;
- your internet browser (you can check your browser version at: http://detectmybrowser.com/).
UHI provides links to external sources of information and may refer to specific Web sites, products, processes or services within this resource. Such references are examples and are not endorsements and whilst every effort is taken to ensure the accuracy of information provided UHI is not responsible for any of the content or guidance. You are advised to exercise caution.
Audio
Video
Reading
Download
Information
External link
Activity
Question
Asterisk
Discussion
Collaboration
Reflection/journal/log
History
Pause for thought
Download a copy of this resource in PDF format.
You can also print individual pages by printing directly from the browser.