Polymer analysis and characterization
Polymers may be analysed and characterized by many different means. Methods may be based on physical properties such as response to waves that may be spectroscopic or mechanical, microscopy, solution properties or thermal characteristics.
Spectroscopic methods are based on interactions between the samples and waves of different frequency and intensity. Commonly used spectroscopic methods include Fourier Transform Infrared Spectroscopy, FTIR, where infrared light is absorbed by different parts of polymer functional groups. It is the different covalent bonds that absorb infrared light. Fingerprints for each individual polymer can be compared to tabulated standard spectra in order to identify polymers. The method is sensitive enough to reveal effects of thermal or chemical manipulations.
Nuclear Magnetic Resonance, NMR, is another spectroscopic method that can reveal structural details about the polymers such as presence and degree of branching, head-to-head coupling, cross-linking and even degree of crystallinity. Determination of the crystalline unit cell is best determined by Wide Angle X-ray Scattering, WAXS, while e.g. interlamellar distances may be determined by Small Angle X-ray Scattering, SAXS. WAXS can also be used to determine the degree of crystallinity in a semicrystalline polymer sample.
Another method, which is very versatile, Differential Scanning Calorimetry, DSC, can also be used to determine the degree of crystallinity as the ratio of the sample's endothermal of melting to the endothermal of the perfect crystal. However, this requires knowledge of the sample's identity and also that the crystallinity does not change during heating up to the temperature where melting starts. DSC also provides Tg data and the crystallization can be studied. A small sample is put in an aluminium pan that is sealed and put on a heating plate. An empty and sealed pan, put on another heating plate is used as a reference. The two pans, either kept in the same or separate ovens, are then kept at the same temperature and the energy needed to accomplish that is registered upon temperature manipulation. Examples of such manipulations are shown in Figure 9 where the Tg is revealed together with the Tm and crystallization temperature Tc and the heats of melting and crystallization.
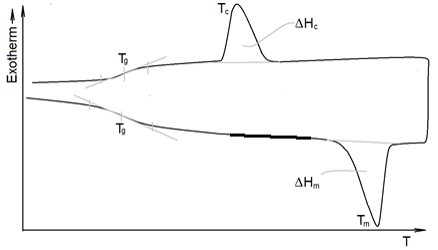
Figure 9. Schematic DSC thermographs of a semicrystalline sample
that has been through a heating and a cooling ramp
© Anders Persson, University of Borås
In cases with samples of low conformational mobility, such as highly crystalline ones, the glass transition may be difficult to detect since the step of altered thermal conductivity seen in Figure 9 becomes insignificant. In that case other methods may be called upon. One such method which is related to the glass transition is Dynamic Mechanical Thermal Analysis, DMTA, where a sample is harmonically deformed and the time lag between applied stress and deformation is monitored as a function of temperature. It is the polymeric materials’ viscoelastic properties and their temperature dependence that is registered. More on this is presented in the section on Mechanical properties and viscoelasticity below. That section will also contain information about tensile testing. Thermal Gravimetric Analysis, TGA, is basically a very sensitive balance contained in an oven with an atmosphere that may be inert or oxidising. In this way volatile components of the samples may be quantified and thermal decomposition kinetics can be described.
It is also essential to have quantitative data on molar mass and molar mass distribution of linear and branched polymers. There are a few options to measure that but the dominating one is Size Exclusion Chromatography, SEC. A diluted polymer solution is injected into a porous column that is flown through by a liquid mobile phase that will bring the dissolved polymers through the column until detected after the column. Higher molar mass also means bigger volume in the solution and the bigger the polymer coils, the fewer column pores will be big enough to contain them and thereby slow them down through the column. Thus the biggest polymers come first and the smallest at the end. A series of well-known molar mass references are utilized to make up a calibration curve to relate the actual samples to.
There are several other characterization techniques that cannot be covered here. Examples of those are different microscopic techniques, density measurements, permeability, dielectric properties, heat and electrical conductivity, etc.
Task: Imagine that you need to determine what polymeric material a specific artefact is made from. What non-destructive method would you choose?
DSC
DMTA
NMR
FTIR

All efforts have been made to ensure materials created by the EDU comply with current accessibility guidelines (JISC: Support for learners with disabilities).
If further assistance is required with accessibility matters please contact the student support section in your academic partner UHI: Accessing learner support.
We welcome any comments on how to improve this unit. Please feel free to pass these on at any time.
If you have any difficulty viewing this resource please contact EDU (edu@uhi.ac.uk) with:
- the name of the resource;
- a description of the problem (please give as much detail as possible);
- the section of the resource where the problem occurred;
- your internet browser (you can check your browser version at: http://detectmybrowser.com/).
UHI provides links to external sources of information and may refer to specific Web sites, products, processes or services within this resource. Such references are examples and are not endorsements and whilst every effort is taken to ensure the accuracy of information provided UHI is not responsible for any of the content or guidance. You are advised to exercise caution.
Audio
Video
Reading
Download
Information
External link
Activity
Question
Asterisk
Discussion
Collaboration
Reflection/journal/log
History
Pause for thought
Download a copy of this resource in PDF format.
You can also print individual pages by printing directly from the browser.